OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Daniel Norwood1, Louis Fleck2, Emre Seyyal2, Anna Michelson2, Gyorgy Vas2,3,*
1FNA Pharma Consulting, LLC; 2Trace Organics Analytical Intertek Pharmaceutical Services; 3VasAnalytical.
Reviews in Separation Sciences. Vol.4. No.1. pages e22003 (2022).
Published online 04 November 2022. https://doi.org/10.17145/rss.22.003 | (ISSN 2589-1677).
*Correspondence:
Vas G. . VasAnalytical, 6 Avalon Ct, Flemington, NJ 08822. USA.
Editor: Marcello Locatelli, University “G. d’ Annunzio” of Chieti-Pescara, Chieti, Italy.
Open-access and Copyright:
©2022 Norwood D et al. This article is an open-access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have declared that no funding and writing assistance was utilized in the production of this article.
Competing interest:
The authors are employed by the above-mentioned organizations and have declared that no competing interests exist.
Article history:
Received 26 July 2022, Revised 15 October 2022, Accepted 17 October 2022.
Abstract
Chemical safety assessment is one necessary step to demonstrate that the polymer used is free from harmful chemicals. The assessment should start with adequate material characterization using state-of-the-art analytical methods, including various chromatography techniques most often hyphenated with mass-spectrometric detection. Since polymers are usually incompatible with direct analysis using chromatography-based techniques, an extraction step is required prior to analytical testing. This paper provides information for practitioners who need to understand how to develop science-based extraction conditions that enable the determination of the polymer composition with respect to volatile compounds. Analytical data sets for three types of polymers (polypropylene, polycarbonate, polybutylene terephthalate, and a rubber elastomer) are presented using three different solvents and three different extraction techniques. The extracts were collected at different times during extraction and analyzed by GC-MS. The differences observed between extraction techniques, conditions, and solvents are discussed to better understand the impact of these parameters in extractable studies.
Keywords
GC-MS, Relative Response Factor, Solvent Impact, Extractables, Leachables.
1.0. Introduction
“Extraction is a process of treating a material with a solvent to remove soluble substances. Extraction is a complex process influenced by time, temperature, surface area to volume ratio (i.e., stoichiometry), extraction medium, and the phase equilibrium of the material” [1]. In addition to those parameters, the solvent and the polymeric material properties also impact the extraction process [2].
The generally accepted approach in designing an extraction study is based on “like-dissolves-like” [3], meaning that the extraction solvent extracts species with similar characteristics. Different solvent parameters have been evaluated and reported for extractable studies, such as polarity, dielectric constant, and logP [4].
The physicochemical properties of the test article or the polymer resin must also be considered when designing extractable studies. The molecular weight of the polymer can impact the extraction efficiency; a higher MW usually slows down the extraction. The Tg of the polymer can also influence the extraction, as higher crystallinity usually results in a lower extraction rate, similar to crosslinking [5]. Extraction studies should be designed so that the polymeric test objects are not degraded, and the extraction process should not impact the integrity of the test article.
Polymeric materials and components used in packaging and delivery systems for pharmaceutical products or medical devices must be suitable for their intended use. They must function properly, adequately protect the pharmaceutical product over its shelf-life and mechanically perform as intended to administer the product to the patient. They must also be mechanically and chemically stable or function properly as a drug-release matrix in combination products. An appropriate and well-designed chemical evaluation of plastic materials can be utilized to support chemical safety and compatibility [6]. The modern practice of assessing extractables and leachables (E&L) can support such evaluations.
Extractables are the chemicals derived from container closure systems (CCS), packaging, or device components, using laboratory solvents of different characteristics and beyond normal use conditions (e.g., elevated temperature, pressure, agitation). Leachables are chemicals derived from CCS or device components that are part of the final drug product under normal storage and patient-use conditions [7]. E&Ls may be associated with a variety of chemicals, or their derivatives, used in the manufacturing of pharmaceutical packaging systems, including catalysts, initiators, additives (e.g., lubricants, antioxidants, anti-tack, and antistatic agents), oligomers with a low degree of polymerization, adhesives, anchoring agents, adhesive resins, and irradiation-induced degradation products and oxides [7,8].
Extractables are potential leachables and thus have only a potential impact on the patient, whereas leachables may have an actual impact. The effective management of contact material-derived leachables in product packaging is critical for the development of many pharmaceutical products, such as parenterals, injectables, ophthalmics, and inhalation and nasal products.
Several regulatory agencies strongly encourage the management of leachables in drug products, which has resulted in multiple regulatory and industry guidance documents that address the expectations for E&L management [1,9-12]. Information about extractables can be used to assist in the selection of a container closure system or delivery system materials for final drug products or finished medical devices [7]. While many existing regulatory guidelines and standards specifically indicate how to plan a process-specific evaluation, the need for further standardization of the E&L assessment process is still debated in the scientific and regulatory communities.
Extractables are most often associated with the chemical composition of the materials; however, chemical additives or processing aids used during material or component manufacturing also need to be considered as part of a comprehensive approach. The pharmaceutical industry has pursued several avenues to obtain comprehensive chemical composition information on the ingredients and processes used to fabricate materials and components employed to construct packaging and delivery systems for pharmaceutical products. These avenues may include requesting information from suppliers, asking suppliers to perform the testing, initiating independent extraction studies on materials, or leaching studies of finished products.
Typically, the burden of acquiring detailed chemical composition information falls to the pharmaceutical manufacturer, who executes or sponsors testing to meet the regulatory requirements. Previously studies have been performed via a consortium of multiple analytical laboratories with E&L assessment capability [13]. These studies aimed to investigate the potential for simplifying E&L assessment protocols. Examples of simplification include using fewer extraction techniques or even just one, or reducing the number of solvents, while still obtaining sufficient information to characterize the polymeric materials. Streamlining these studies might lead to a more effective materials selection process [13]. It was expected that extractables assessment studies, when accomplished, would provide qualitative and semiquantitative information on extractables consistent with the identity of the material of construction and its additive composition. Although a consensus was reached on scientific principles and analytical techniques, working groups and laboratories across the industry proposed different protocols to perform E&L assessment for different polymeric systems. These proposals included extraction conditions and analytical workflows consisting of physicochemical analyses, spectroscopic techniques, and advanced analytical techniques that enable the identification and characterization of a wide spectrum of organic compounds and inorganic elemental impurities at trace (ppb or ppt) levels [14].
Generally, material selection, when possible and relevant, would also include information from the supplier, for example, regarding processing agents and washing conditions, which would also be some of the analytes investigated in extraction studies. One of these benchmark studies, the ELSIE pilot program [13], did not use industrially fabricated parts or components, but rather the resin (i.e., the material of construction) and the resin processed via a known protocol (with no processing agents). The effect of processing on the extractables profile from mechanical and thermal stress was evaluated as part of the ELSIE protocol and discussed with industrial examples in a separate paper [15].
Several round-robin studies were published targeting multiple types of polymeric materials, extraction techniques, solvent combinations, and other practical variables [13,15-17]. Those studies were conducted by different analytical laboratories, using various approaches to perform the extractions and analyze the extracts. Despite the great effort to design and standardize those studies, drawing a general conclusion about the materials, extraction techniques, and conditions is difficult. Although these publications share significant levels of detail about different extraction methods [13,16] and solvents and propose best practice approaches for analyzing the extracts, they lack justification for selecting the extraction conditions. The extraction conditions, which include the type of extraction, the duration, and the temperature, have a significant impact on the outcome of the extraction study as described in the United States Pharmacopeia (USP) standard [10].
In this study, three materials were selected for evaluation: a polypropylene resin, a PBT Xenoy resin, and a chlorobutyl rubber stopper. The stopper was a finished sterilized packaging component. After preliminary experiments that focused on ester-forming potential, evaluation of dielectric constant, and swelling with a variety of solvents, three extraction solvents of different polarities and chemical characteristics were selected. Isopropanol (IPA) is a relatively polar organic solvent with a polarity index of 3.9 and a dielectric constant of 19.92; cHex is a non-polar solvent with a polarity index of 0.2 and dielectric constant of 2.02, and dichloromethane (DCM) is an intermediate solvent between alcohol- and hydrocarbon-type solvents with a polarity index of 3.1 and dielectric constant of 8.93. The solvents were combined with three extraction techniques: reflux at atmospheric pressure, shaking with a closed vessel (which generates a slight over-pressure during the experiments), and a pressurized vessel. The conditions used in the experiments differed from those listed in ISO 10993-12 [18]; therefore, they may not be appropriate for the toxicological risk assessment of medical devices.
The resulting extracts were subjected to gas chromatography-mass spectrometry (GC-MS) analysis, which targets volatile and semi-volatile extractable species. Since the analysis focused on volatile species, the conclusions made here are not appropriate for a full-scale assessment of the extractables. The measured asymptotic concentration levels were used to identify the practical end-point of the extraction, which is appropriate to characterize polymeric materials for pharmaceutical packaging [10].
2.0 Experimental
2.1 Materials, chemicals, and standards
Previous studies have evaluated low-density polyethylene (LDPE), polyvinyl chloride (PVC) (multiple studies), polyurethane, cyclic olefin copolymer (COC), polycarbonate, and isobutylene rubber [13,15-17]. In this study, the following polymeric materials were examined:
- Chlorobutyl-rubber grey-colored septa (West 4432/50 coated with BH2-40 and Flurotec (P/N 19700022) WPS S10-F451 4432/50G B2-40WESTARRS1M/PK), sterilized;
- PC-PBT Xenoy 6370 (polycarbonate-polybutylene terephthalate) glass-fiber reinforced black pellets;
- Polypropylene (PP) pellets, natural no colorants (Polypropylene Plastic Resin Pellets Natural Injection Molding PP Impact co-polymer Flint Hills Resources, Lot. No. AP61123-HS).
The final extraction solvents for the study were selected after the impact of swelling and alcohol-ester formation were evaluated. After analyzing the data from the preliminary experiments (see other sections for detailed descriptions), three extraction solvents were selected for use: 2-propanol (IPA), cHex, and DCM. These extraction solvents were acquired from Millipore Sigma (St. Louis MO, USA) with trace analysis purity. The additional solvents used for the solvent selection evaluation process, specifically, methanol, ethanol, ethyl-acetate, n-hexanes, heptane, iso-octane, and toluene, were acquired from Millipore Sigma in HPLC or GC grade. To estimate the quantity of the extracted analytes, a multi-component mixture of internal standards (EPA B/N mixture) containing d5-nitrobenzene, 2-fluorobiphenyl, pyrene-d10, and terphenyl-d14, was purchased from Restek Corporation and used at a 1 µg/mL spiking level.
2.2. Extraction conditions
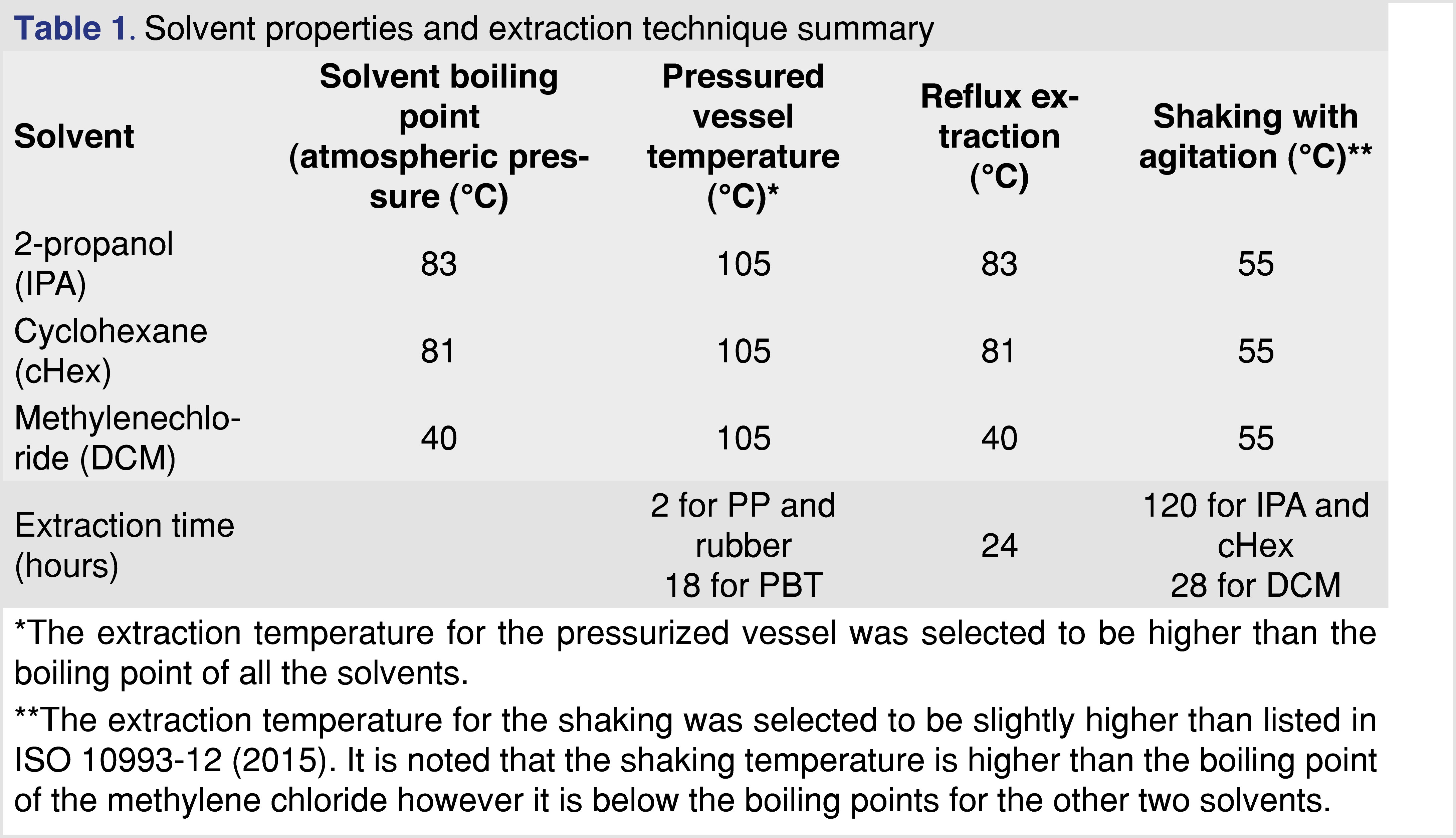
The study focused on volatile and semi-volatile analytes. Based on the previously reported data, the “extractable profile” for the studied materials was mostly organic [16], and when water was used as the extraction medium, a low level of extractables was observed. Therefore, water was not used in this study to optimize the extraction conditions. The properties of the solvents are listed in Table 1.
The extraction studies were performed using three extraction techniques:
- Closed-vessel pressurized extraction, where the samples were placed in pressure-resistant stainless-steel extraction vessels with a 50-mL internal volume. Approximately 3 grams of material were used for the extraction with 30 mL of solvent (10 mL solvent for each gram of polymer). The closed extraction vessels were placed into a calibrated oven for 18 hours at 105°C. (For dichloromethane, the pressure in the vessel is approximately five 5 times atmospheric pressure.) Intermittent sampling was performed at 0.5, 1.0, 1.5, and 2.0 hours after the extraction started. For each intermittent sample, the vessels were rapidly cooled (less than 2 minutes) to ambient laboratory temperature using a liquid nitrogen cooling bath, and 0.25 mL of extract was removed (in triplicate). The cHex and DCM solvents impacted the PP and the rubber samples by swelling the materials as a result no sampling was performed at the end of the extraction period as no solvent was recoverable. These solvents did not impact the PBT polymer.
- Reflux extraction, where the polymer samples were placed into an open glass vessel (1 g to 10 mL polymer to solvent ratio), the solvent was added, and a vapor condenser unit was connected. The vapor condenser unit was cooled via a recirculating cooling unit maintained at 8°C to ensure proper condensation of the solvent vapor. Extraction was performed at the boiling temperature of the solvent. (The boiling points of the solvents are listed in Table 1.) Intermittent sampling was performed at 1, 2, 5, 8, and 24 hours of extraction time with 0.25 mL removed at each time point (in triplicate). The solvent loss during the reflux process was less than 10% over 24 hours.
- Closed-vessel agitation (shaking), where the polymer samples were placed into a medium-pressure-resistant sealed glass vessel, and a 1:10 ratio of sample to solvent was added. The type of vessel used for the extraction can hold medium pressure for a limited time. The samples were kept in a heated shaker at 55°C and 150 rpm. Sampling during the extraction was performed at 4, 20, 28, 48, 72, and 120 hours. The sampling was performed by taking 0.25 mL of the extract in triplicate. Before sampling, the vessels were cooled to ambient temperature to minimize the potential loss of volatiles.
The extraction vessels used in the study were selected to address volatile and semi-volatile organic extractables. The glass and the stainless-steel vessels are compatible with hyphenated chromatographic methods. However, due to their composition, they would generate elevated levels of Na, K, B, Si, Fe, Cr, Ni, and Co; the extracts were not intended to evaluate elemental impurities. The other reason the elemental impurities were not monitored is that the organic solvents used in this study are a poor choice for extracting elemental impurities and require a special method to introduce the sample to the plasma source (solvent exchange or dilution).
2.3. Justification for the solvents used in the study
The regulatory and industry expectations regarding extractable studies are that solvents or solvent systems with different polarities and chemical characteristics should be used to extract the widest possible range of chemical species [7,8]. Solvents should be selected based on scientific rationale, and the scientist should be aware that the solvent can alter the outcome of the extraction study. It is well known that protic solvents, such as ethanol or isopropanol, can react with acidic extractables to form esters; however, very limited data have been published [19]. Our data show that ethanol produces the highest conversion rate among the three alcohols selected for evaluation (methanol, ethanol, and isopropanol).
To study the esterification effect, a PP material was used, with three solvents in a pressurized vessel for a 2-hour extraction period. After the extractions were completed, the vessels were cooled, the samples were injected into a GC-MS, and the differences between the samples were evaluated.
The conversion rates were calculated based on the following equation:
Equation 1:

For the DCM extracts, the sum of the methyl, ethyl, and isopropyl esters was determined and used as a control, as those esters are present in the non-extracted polymer, and DCM is inert with respect to ester formation. Therefore, no further ester formation is expected.
The results for the formation of palmitic and stearic acid esters show that while IPA has a 10%–14% conversion rate, methanol has 28%–36% conversion, and ethanol shows a 49%–67% conversion, even for this relatively short extraction time.
A similar phenomenon was observed for 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid, as the formation of the different alcohol-esters was observed at different levels in the extracts. One of the most often observed degradation products of Irganox-1010 is 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid, reported earlier in the literature [20,21]. As it has an acid functional group, it is prone to react with alcohol extraction solvents. Under the conditions used for this evaluation, the level of ethyl-ester was elevated by almost 800-fold when ethanol was used for the extraction compared to the DCM control extraction. In comparison, the elevation of the isopropyl-ester was 95-fold, and the elevation of the methyl-ester was 8.5-fold.
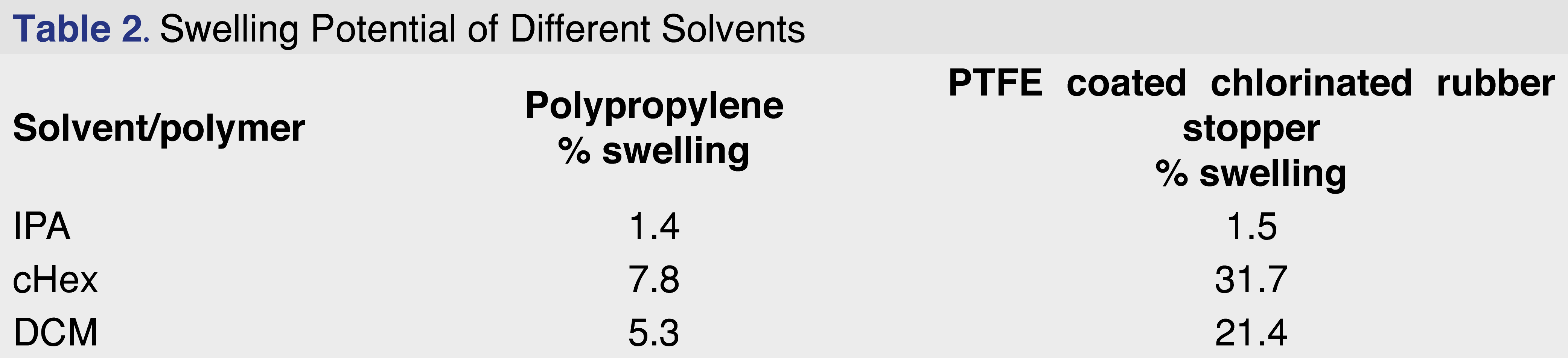
Based on the ester formation data, isopropanol was selected as the protic extraction media for the studies, DCM as a mid-polar solvent, and cHex as an apolar solvent. The impact of solvent swelling from a wide range of solvents is discussed in the results and discussion sections of the paper (see Table 2 and Table 3).
2.4 Internal standard used for the study
A multi-component mixture of internal standards (EPA base-neutral), which includes four compounds with different chemical properties, chromatographic retention times, and chromatographic behavior, was selected. The nitrobenzene-d5 provides a low response, is relatively polar, and elutes early, 2-fluoro-biphenyl elutes at the middle of the chromatogram, and pyrene-d10 and terpenyl-d14 elutes at the end of the chromatogram. Internal standards (IS) were applied to compensate for the variation in the injection process, and the extracted analytes were quantitated against the average response of the spiked internal standards. The internal standard was spiked into each extract at a 1 µg/mL (1 ppm) level, equivalent to 10 µg/g of the polymer material since a 10 mL to 1 gram solvent-to-polymer ratio was used. The 1 ppm level is a comfortably low concentration to address the extractables at the early stage of the extraction process and provides sufficient signal for the analysis. All the extracted chemical species were evaluated at or around 0.2 analyte/IS ratio.
2.5 Instrument conditions
2.5.1 GC-MS conditions
The sample extracts were analyzed with GC-MS, which consisted of a Thermo Scientific Trace 1310® high-resolution gas chromatograph (GC) with a TSQ9000 triple quadrupole detection system (MS). The GC was equipped with a PTV injection system, used in a programmed temperature splitless mode at 40°C to 260°C with a 12°C/sec heating rate and 0.75 min of splitless time. For the chromatographic separation, a Restek Rxi 5 MS column (40 m x 0.18 mm with 0.18 µm film thickness (β=250)) and He carrier gas (Ultra High Purity, less than 1 ppm total impurities) at 1.1 mL/min constant flow were used. The temperature program was 50°C (1-minute hold) to 325°C at 10°C /min (3-minute hold). Electron ionization was performed at 70 eV energy and 50 µA current with an ExtractaBrite™ ion source operated at 275°C. A 0.5 µL volume of sample was injected for analysis. Data acquisition was performed in 50-550 m/z range using a 5 scan/sec scanning rate.
The samples were injected in triplicate for each extract, and the average of the triplicate testing was plotted. System suitability criteria for the analysis was that the signal-to-noise of the lowest responding analyte, nitrobenzene-d5, must be at least 50:1 RMS for each extraction solvent and condition.
For additional confirmation of the extracted chemical species, a Thermo Scientific GC-Orbitrap® High-Resolution Accurate Mass System (GC-Orbi) was used to confirm the identity of the extractable species. Except for using a shorter chromatographic column for the separation (Restek Rxi 5 MS 30 m x 0.25 mm with 0.25 µm film thickness (β=250)), the chromatographic conditions were similar to those used above. The high-resolution detection system was used at the 60,000 resolution setting with a mass error of less than 1 ppm, using similar ion source conditions to those listed above.
Figures and Tables
[Click to enlarge]
2.6 Method performance for analyzing the extracts
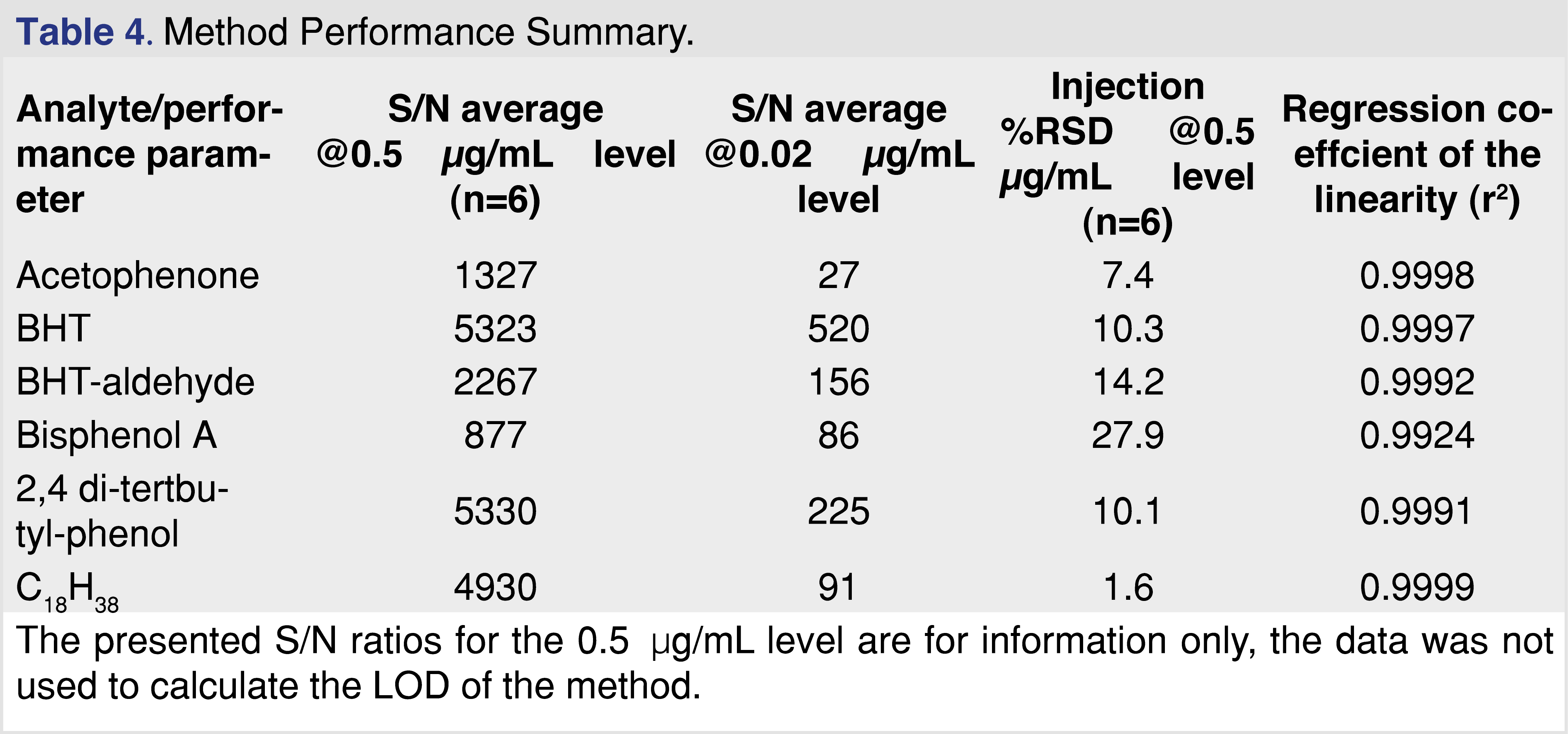
In order to be confident in the GC-MS analysis of the extracts, the method was evaluated using standard solutions of some of the identified extractables, specifically, acetophenone, 2,4-di-tert-butyl-phenol (2,4 DTBP), bisphenol A, BHT, and BHT-aldehyde. As numerous hydrocarbons were observed, a saturated linear hydrocarbon of C18H38, which eluted in the middle of the chromatographic range, was also evaluated. The following performance parameters were evaluated; limit of detection (LOD), based on the calculated S/N ratio at 0.02 µg/mL, injection precision based on the %RSD injection at 0.5 µg/mL level, and linearity in a range of 0.5–5 µg/mL. The following results were obtained for the different analytes (Table 4). Based on the 0.02 µg/mL concentration standard, it can be confirmed that the method LOD is lower than 20 ng/mL for all of the studied analytes.
Since every sample was spiked with a multi-component IS mixture, the %RSD for the response ratio was also calculated, and the values were in a range of 8.7%–19.4% at the 1 ppm spiking level for the different analytes (the lowest responding analyte, nitrobenzene-d5, showed the highest injection %RSD). This level of injection %RSD meets the published requirements for this type of testing [22,23].
Based on this performance evaluation, the analytical method is fit for estimating levels of the extractables and can be used to compare the extraction processes. In general, more extensive full quantitative method validation was not performed, as this activity is usually not required for initial extraction studies (i.e., controlled extraction studies) [13,15-17,23].
3.0. Results and Discussion
3.1 Polymer swelling
During the experiments, the cHex and DCM solvents caused the PP and rubber elastomer samples to swell. In some cases, the extraction solvent was unrecoverable; therefore, the effect of this swelling on extraction performance was evaluated. Swelling is defined as the penetration of a solvent into the polymer network, which causes an abrupt volume change, moving the boundary from the unsolvated glassy region to the solvated region and expanding the rubbery domain [24]. The absorption of liquids leads to changes in the mechanical properties of the swollen material and may create extra pressure when it occurs in confined spaces, which results in various deformations of the swollen material (i.e., surface creases and wrinkles). This process may also significantly alter the adsorption–desorption properties of adsorbates [25]. As the solvents penetrate the polymer network, the chemical additives present in the polymer network become more accessible to the solvent, and diffusion into the solvent network will be enhanced and accelerated.
The PBT Xenoy resin showed no swelling in IPA, cHex, or DCM, while the rubber elastomer showed the highest degree of swelling in cHex and DCM. Overall, cHex showed the highest degree of swelling for both the PP and the rubber elastomer. The conventional definition of the degree of swelling is described as the percentage of weight increase of the polymeric material. This definition works very well if different materials are compared using the same solvent; however, different solvents were used on different materials in this case. Because the density of DCM is 72% higher than that of the cHex, the weight difference may not be the best parameter to evaluate the swelling rate. Therefore, the percentage of volume change for the evaluation was used, as described in Equation 1.
The swelling data are presented in Table 2. Swelling is defined as the percentage of the volume change after solvent exposure for 24 hours at room temperature using 10 mL of solvent for each gram of polymer.
Equation 2:
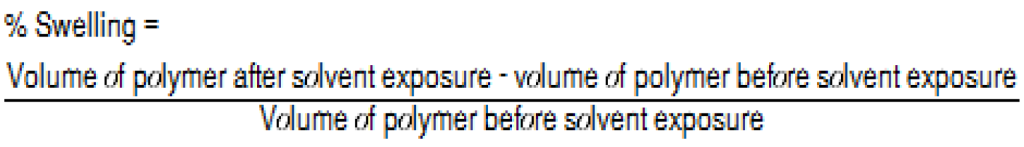
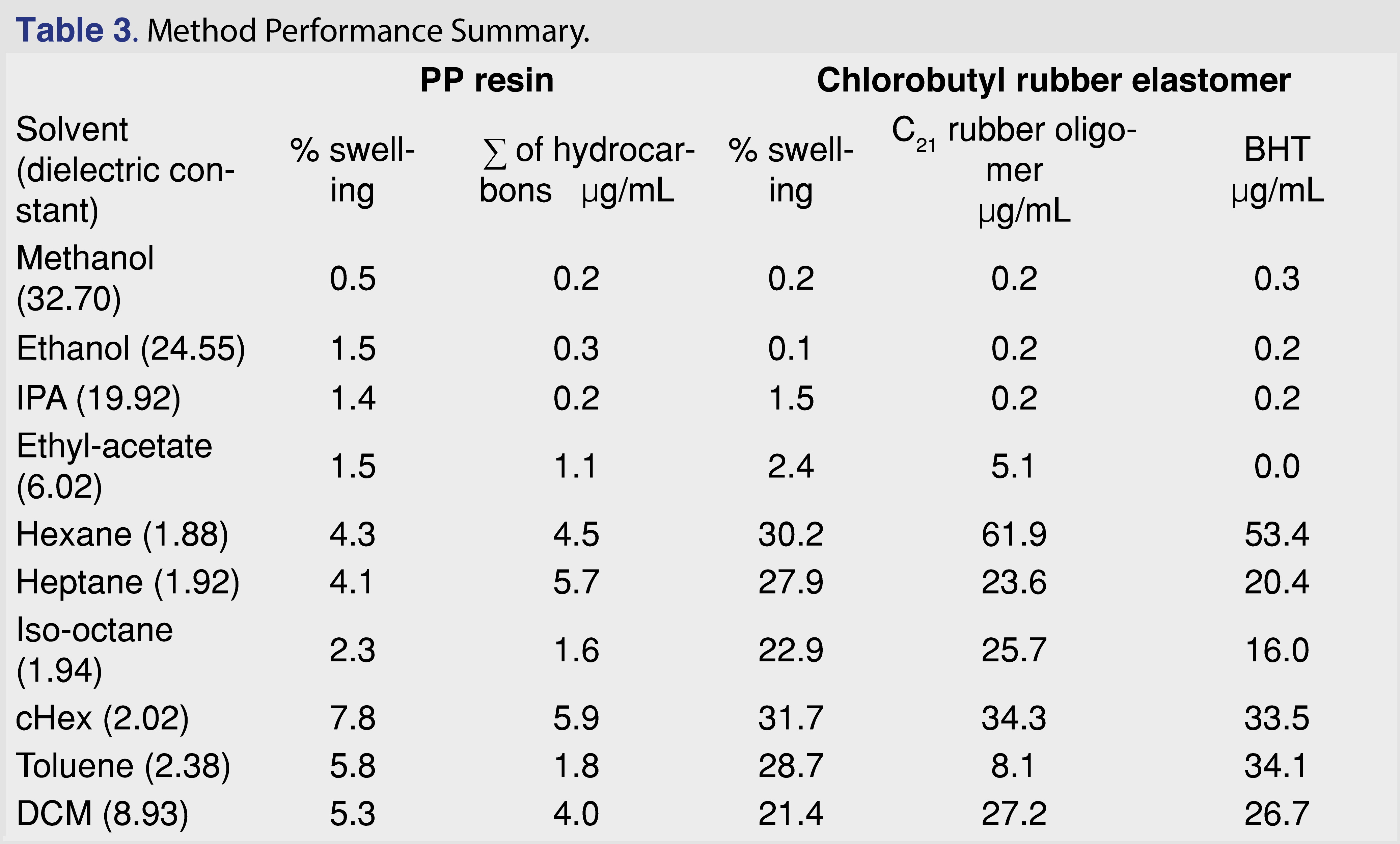
In order to better understand how the solvents impacted the levels of extracted analytes and to determine the correlation between the swelling power of the solvent and the observed extractables levels, an experiment with 10 different solvents was performed. The organic solvents covered a range of polarity and compound classes, including alcohols, hydrocarbon-type solvents, aromatic solvents, an ester, and a halogenated solvent. The solvents are listed in Table 3. The PP resin and the chlorobutyl rubber stopper were used as is, without cutting or grinding, at a 10 mL/g solvent-to-polymer ratio, at room temperature with no agitation or other, more aggressive, manipulation. After 24 hours of soaking, the polymers were removed from the solvents, the volume of the polymers was measured, and the % swelling was calculated using the equation above. The extracts were spiked with the internal standard to support quantitative evaluation and analyzed by GC-MS. For the PP resin, the sum of the hydrocarbons was calculated in µg/mL, and for the rubber elastomer, the level of the C21H40 butyl-rubber oligomer and the BHT were calculated and are presented in Table 3.
The data show a correlation between swelling and the BHT level. However, this does not demonstrate causation, as other solvent properties (such as logP and polarity) can play an important role. The degree of swelling was significant for toluene, but the amount extracted is high for BHT and low for the hydrocarbon-type analytes (i.e., C21H40 oligomer from rubber and hydrocarbons from PP).
The trend is not as strong for the sum of hydrocarbons in PP or the C21H40 butyl-rubber oligomer in the elastomer. The observed levels for the selected extractables were below the saturation point of the target analytes, as it was possible to make a 5 mg/mL concentration solution for BHT and C8–C40 hydrocarbons for each of the solvents. Thus, the overall solubility of the BHT and the hydrocarbons (especially in alcohols) has no impact on the observed results. (The reference standard for the C21H40 rubber oligomer has limited availability and is prohibitively expensive; however, it is chemically a hydrocarbon, and the mass spectral fragmentation is similar to hydrocarbons.)
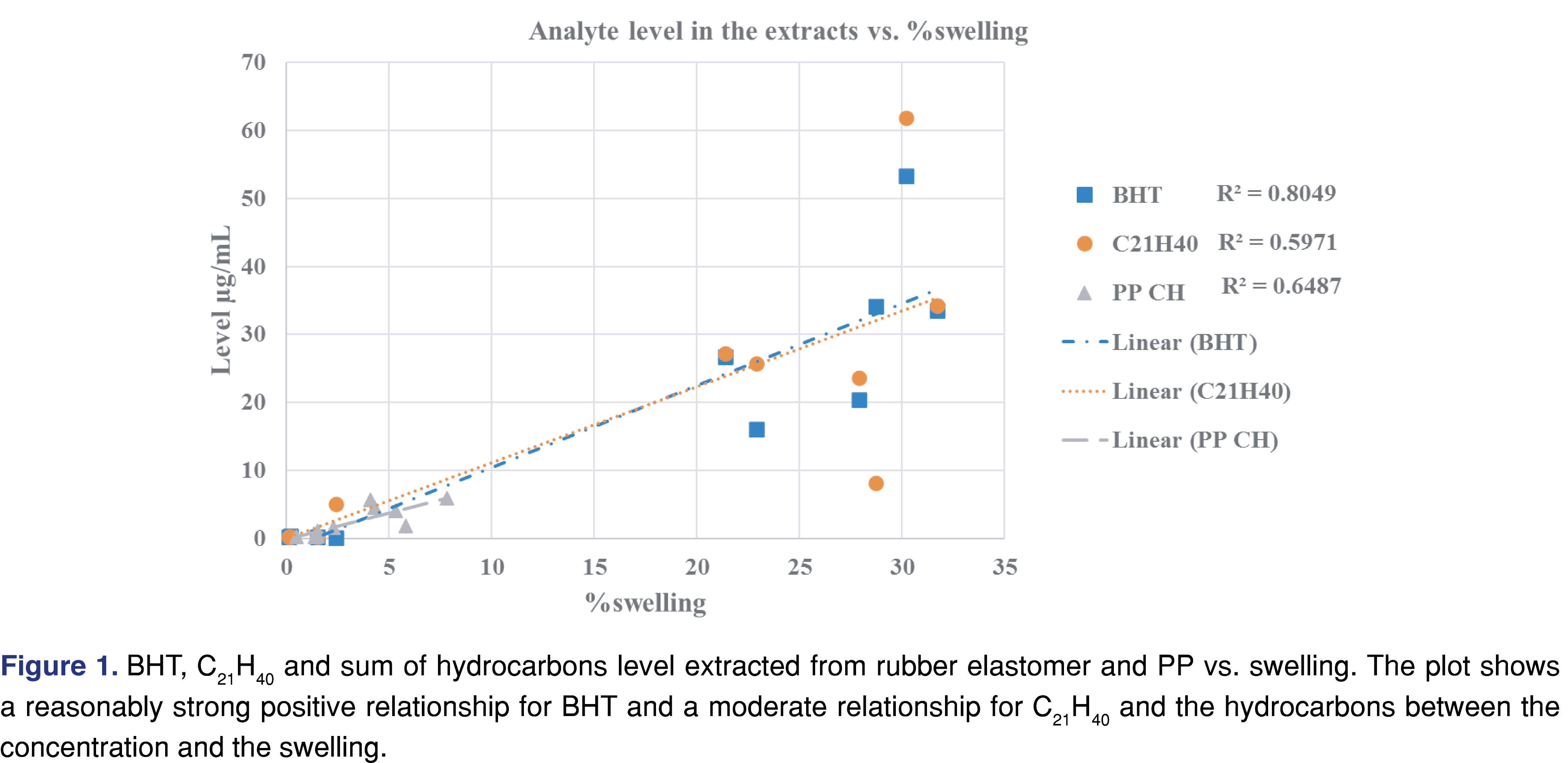
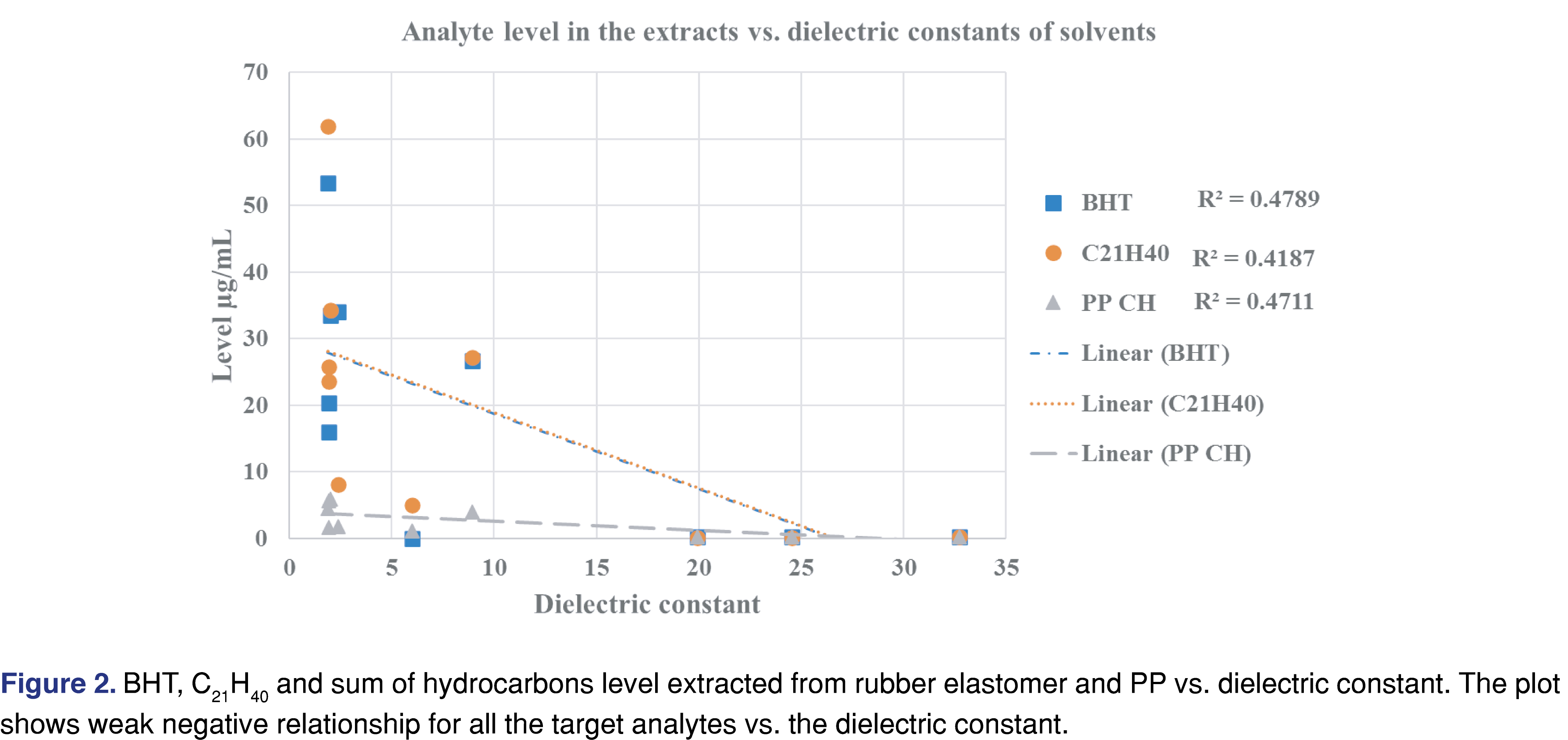
Generally, a fair or medium correlation was observed between the swelling property of the individual solvent and the observed level of analyte in the extracts (Figure 1). However, it is very difficult to predict the “best-performing solvent” for a certain type of polymeric material. This experiment can help narrow the solvent choices and determine an appropriate solvent/polymer ratio to avoid full absorption of the solvent by the polymer matrix. A recent study has claimed that the dielectric constant of the solvent and the extracted level of the analyte were correlated [26]: a reasonable correlation between the dielectric constant and the level of extractables was observed. However, the study was limited to relatively polar matrices with high dielectric constant values (the dielectric constant values ranged from 45 to 78) and therefore had limited applicability to predict the outcome of an extraction study where multiple solvents are used. No correlation was observed in our work, which includes apolar solvents (see Figure 2). If such a prediction tool is implemented in the future for industry use, it should be based on a wide range of solvents, target analytes, and polymer matrixes, similar to the tool developed by United States Food and Drug Administration Center of Device and Radiological Health (FDA CDRH) [27].
3.2 Result of the extraction studies
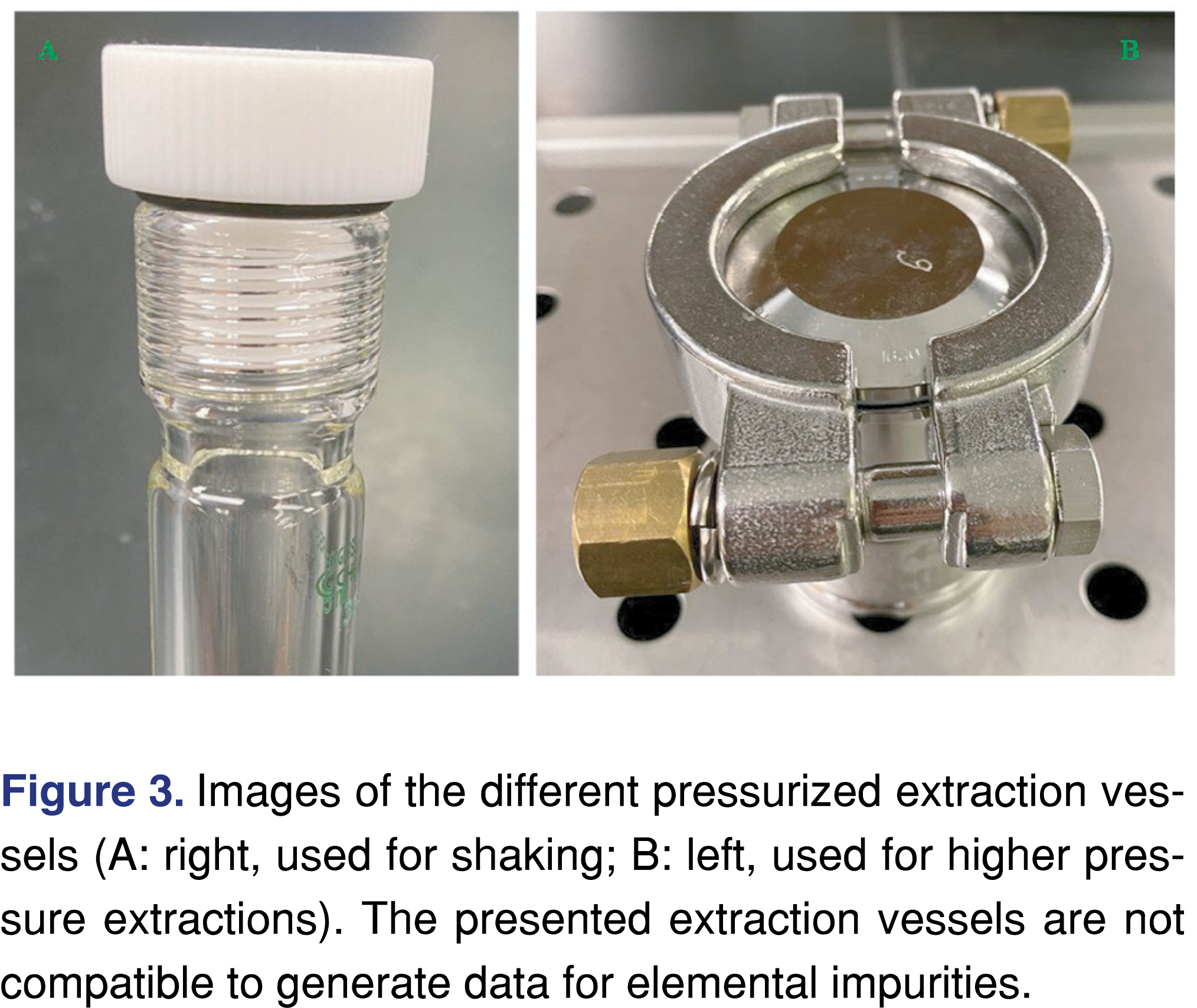
The extraction techniques and solvents used in this study are listed in Table 1. All the chosen extraction techniques are included in USP General Chapter <1663>. Therefore, they are considered standard for extraction studies [6,8]. There are technical issues associated with these extractions: where elevated pressure is associated with the extraction technique, the integrity of the extraction system can be compromised. Figure 3 shows the type of extraction vessels used in this study (vessel A was used for the shaking experiments, while vessel B was used for pressure vessel extractions). The glass vessel with the black elastomer seal (vessel A) is capable of holding a medium pressure (our estimate is consistently holding double the atmospheric pressure) and was suitable for IPA and cHex; however, it was not suitable for the DCM extraction as after 28 hours, a significant loss of analytes was observed. Vessel B uses a non-elastomeric PTFE seal, and it is capable of dealing with higher pressures (more than 20 times the atmospheric pressure) and was, therefore, suitable for all the solvents used in this study, even at high extraction temperatures.
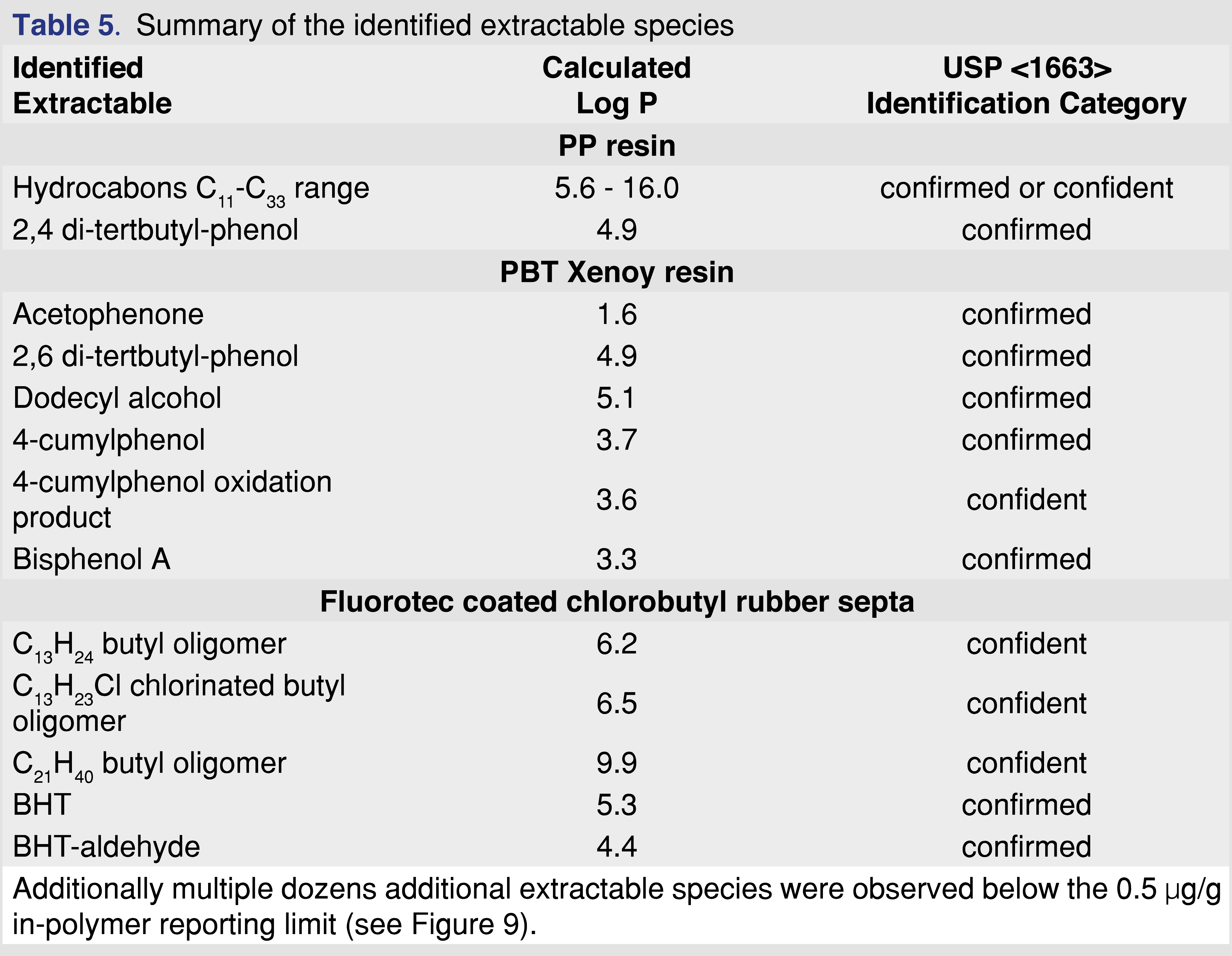
The analysis of the extracts was designed to be performed only with GC-MS for two reasons. First, the low molecular weight species usually migrate faster (inversely proportional to the square root of the molecular weight, Graham’s Law), and therefore asymptotic levels could be reached more quickly. Second, the identification process for small molecules, using GC-MS is more straightforward than for non-volatile species using analytical techniques such as LC-MS (liquid chromatography–mass spectrometry). This facilitated the identification of all observed extractables (at least to the confidence level required according to USP <1663> [10]) above the reporting limit and avoided the often observed unknown extractables observed in similar studies [13,17]. The extractables are listed with associated identification categories in Table 5.
3.3 Identification of extractables
The identified extractables above the 0.5 ppm reporting level from different materials are listed in Table 5. The identifications were accomplished based on authentic reference materials (when available). For extractable species with no available reference standard, identification was accomplished based on a NIST (version 2.4 2020) library search (a library match factor of at least 850), expert spectral interpretation, and elemental composition based on high-resolution accurate mass measurements. The study focused only on evaluating volatile extractables. For the identification of volatiles in industry, the best practice was followed as indicated above. The identification steps described above were followed according to USP General Chapter <1663> [10].
3.4 Analytical results for polypropylene (PP) resin
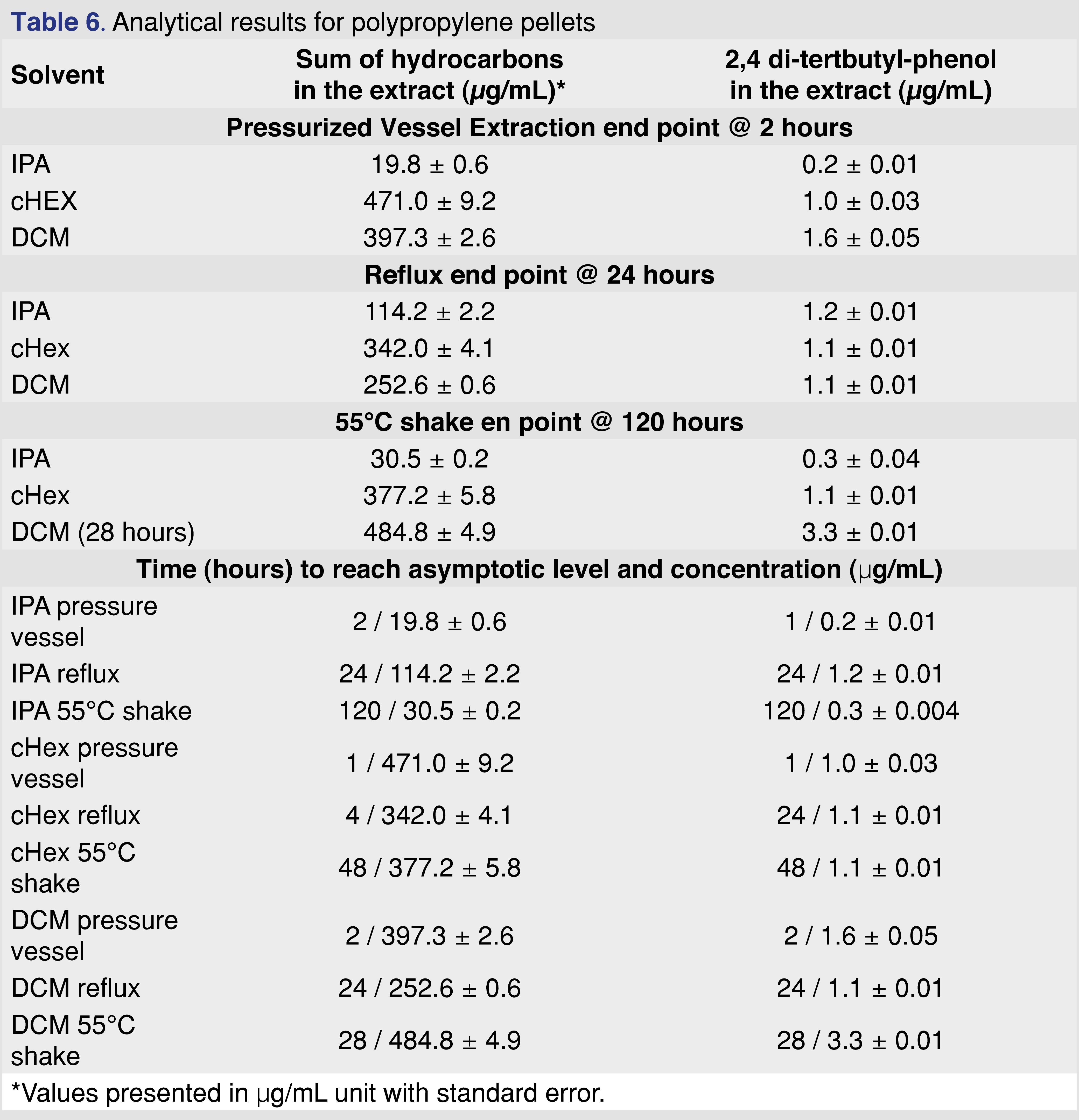
Polypropylene is one of the most commonly used polymers for pharmaceutical packaging systems and medical devices. It has good mechanical and chemical properties, good biocompatibility, good resistance to heat sterilization, and can be recycled relatively easily [28]. PP resin pellets (Polypropylene Plastic Resin Pellets Natural Injection Molding PP Impact co-polymer, Flint Hills Resources Lot. No. AP61123-HS) were used for this study at a ratio of 1 gram of resin to 10 mL of extraction solvent. The PP resin shows very little (almost negligible) swelling in IPA and moderate observable swelling for cHex and DCM (Tables 3–4).
The major extractables for all solvents and conditions were different chain length saturated and unsaturated (a single double bond is the most common) hydrocarbon species and an Irgafos-168 degradation product, 2,4 di-tert-butyl-phenol, which is a single “arm” loss of the Irgafos-168 (Irgafos-168 was also detected; however, it was not reported because the level was below the 0.5 µg/mL reporting limit. Another common extractable detected below the reporting limit was the methyl ester of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid, a commonly reported volatile degradation product of Irganox 1010 [21]. The ester formation discussed in a separate chapter also indicates the presence of Irganox 1010 in the polymer matrix. The results of the extraction study are summarized in Table 6.
The sum of hydrocarbons includes saturated-alkane hydrocarbons with chain lengths of C11– C33. The solubility of long-chain hydrocarbons (above C=30) is higher than 5 mg/mL in each of the solvents. Therefore, the solubility and analyte saturation have minimal impact on the observed results, even for IPA. The solubility of the 2,6-di-tert-butyl-phenol is higher than 5 mg/mL in each of the solvents, as stock standards were prepared in each of the solvents at 5 mg/mL concentration, and no precipitation was observed.
The data show that extraction solvents are not equivalent (as was expected); however, noticeable differences were observed between the extraction techniques. The time to reach the asymptotic level of extractable analyte varied from 2 to 120 hours, depending on the extraction technique. The other noticeable difference was the maximum observed level of extractables, which covered a relatively wide range for the different techniques and solvents. For example, the observed maximum level for 2,4 DTBP was in the range of 0.2–3.3 µg/mL, and the hydrocarbon extractables were in the range of 20–486 µg/mL.
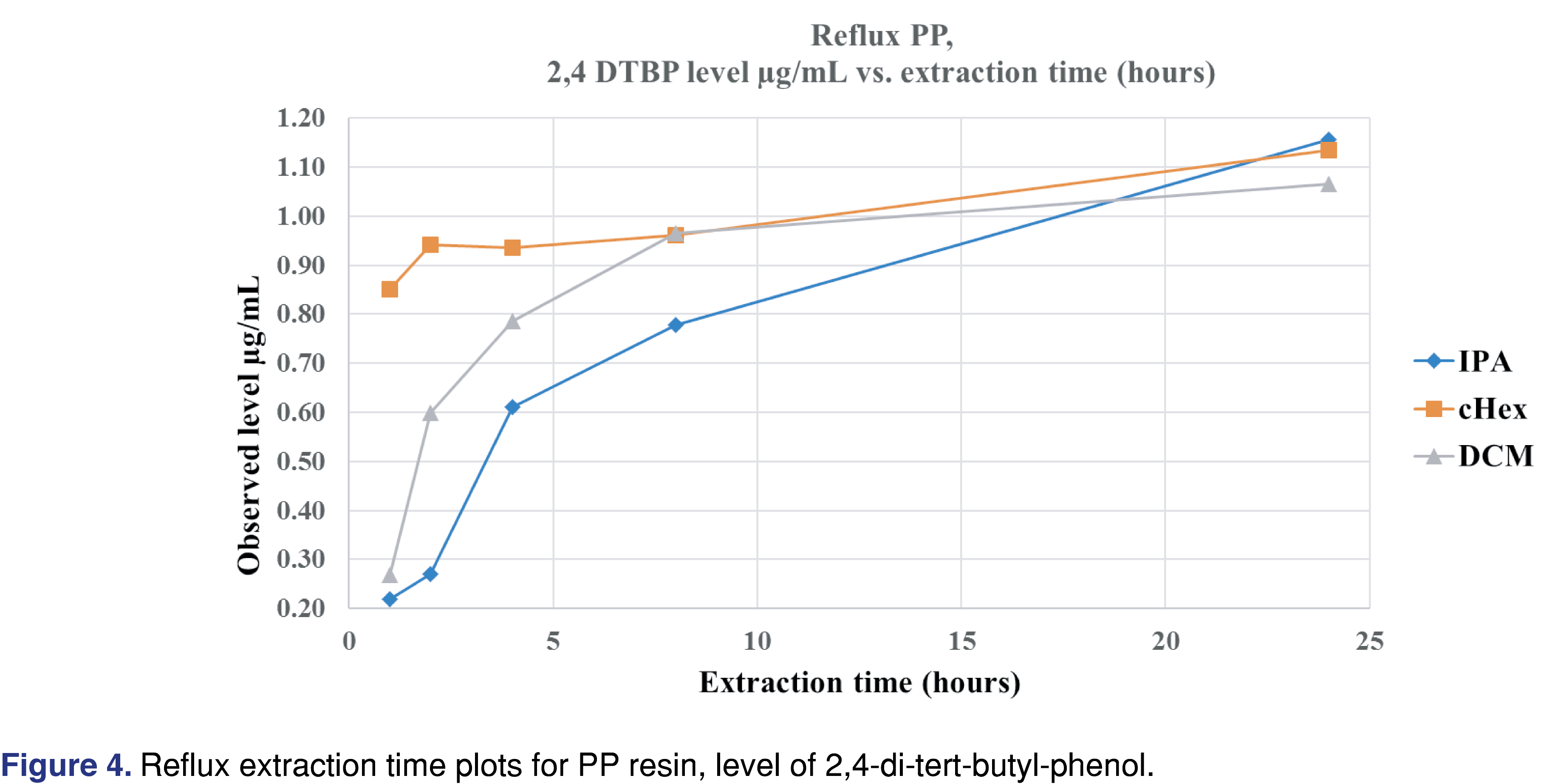
Figure 4 shows that after 12 hours of reflux extraction, the 2,4-DTBP level is close to the observed maximum for DCM and cHex. For isopropanol, the observed concentration is at 70% of the maximum after 12 hours. Based on these data for the PP resin, IPA is the least effective solvent, and this observation aligned with the fact that the IPA swelled the resin the least (lowest % of swelling). Shaking with elevated temperature takes the longest time to reach equilibrium. However, some of the solvent and extraction conditions compromised the integrity of the polymer. In some cases, those aggressive conditions cannot be used for evaluation (e.g., cHex with pressure vessel). Even with specialized extraction vessels (Figure 3A), the extraction temperature should be below the boiling points of the solvents; otherwise, the loss of volatile analytes is possible. Therefore, it is necessary to use the lowest possible temperature to minimize the loss of volatile species without negatively impacting the extraction time. Below the boiling point of the extraction solvents, shaking is an easily executable solution. In contrast, the pressurized vessel is a low-cost alternative to ASE (accelerated solvent extraction) and provides results in a timely manner.
3.5 Analytical results for PBT Xenoy resin
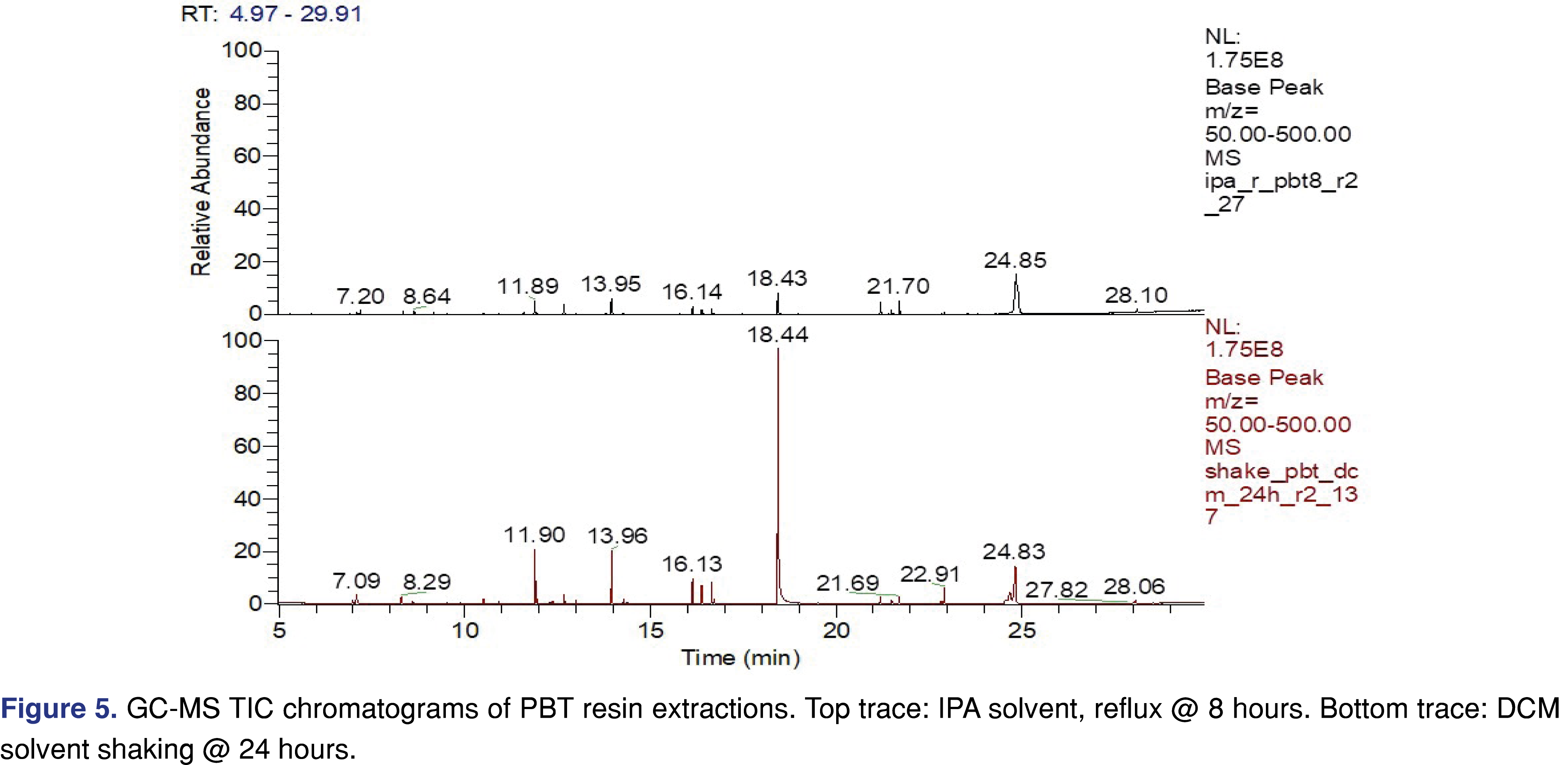
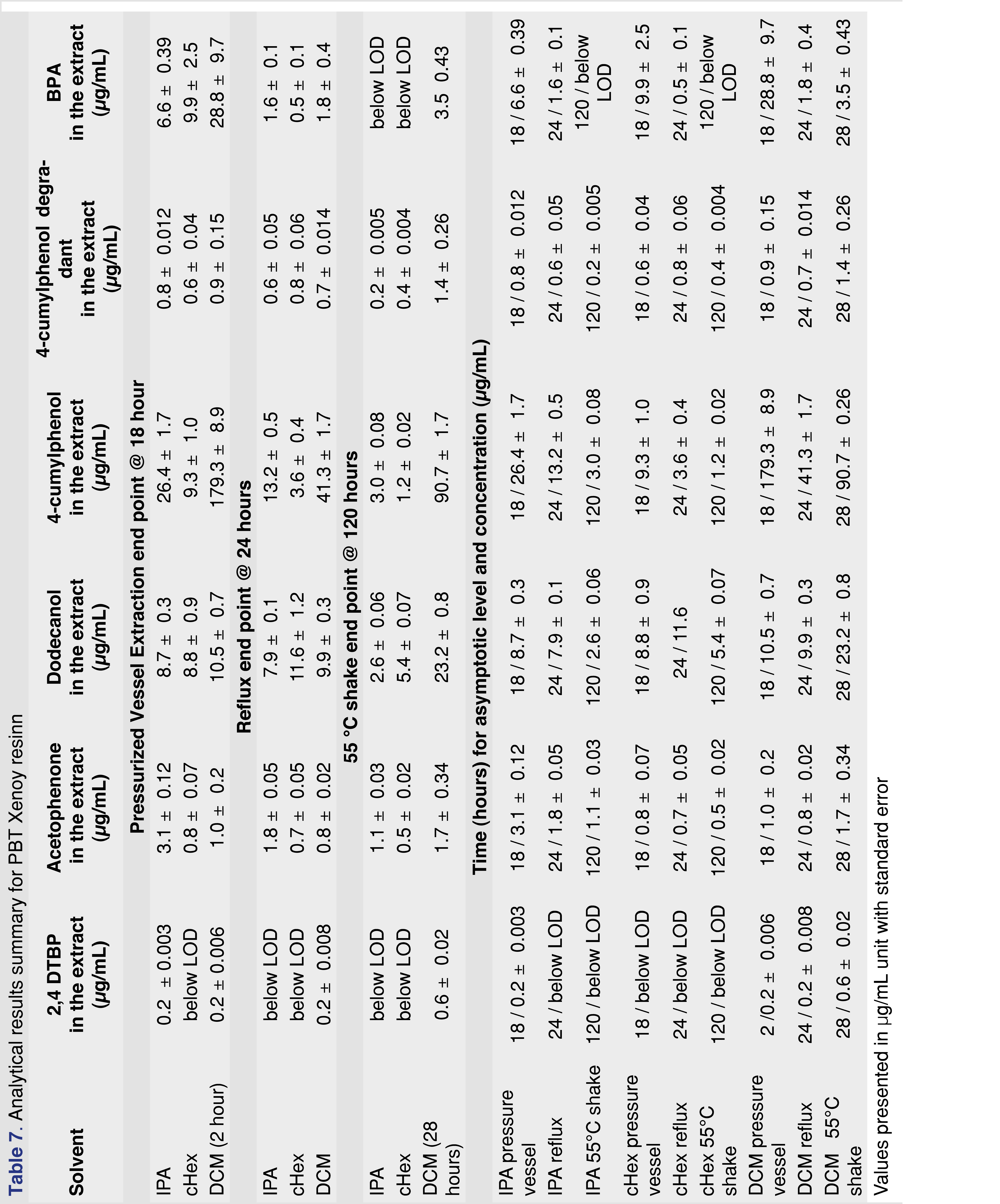
PBT Xenoy resin pellets (PBT Xenoy 6370 polycarbonate-polybutylene terephthalate glass-fiber reinforced black pellets) were used for this study at 1 gram of resin to 10 mL of solvent, similar to what was used for the PP resin and chlorobutyl rubber. The PBT resin showed very little (almost negligible) swelling in the solvents. This is important for the pressure vessel method, as all the solvents were recoverable even after 18 hours of extraction. Therefore, data collected after 18 hours in the pressure vessel are presented for the PBT resin. The major extractable for all the solvents and all the conditions was 4-cumylphenol. The major use of 4-cumylphenol is a chain terminator for polycarbonates and other phenolic resins; detecting this compound as an extractable is somewhat expected [29]. The other abundant extractable was identified as 1-dodecanol (lauryl alcohol), often used as a lubricant or defoaming agent in polymer synthesis processes. Representative total ion chromatograms (TICs) for DCM and IPA extraction solvents are presented in Figure 5, showing significant differences between these two solvents.
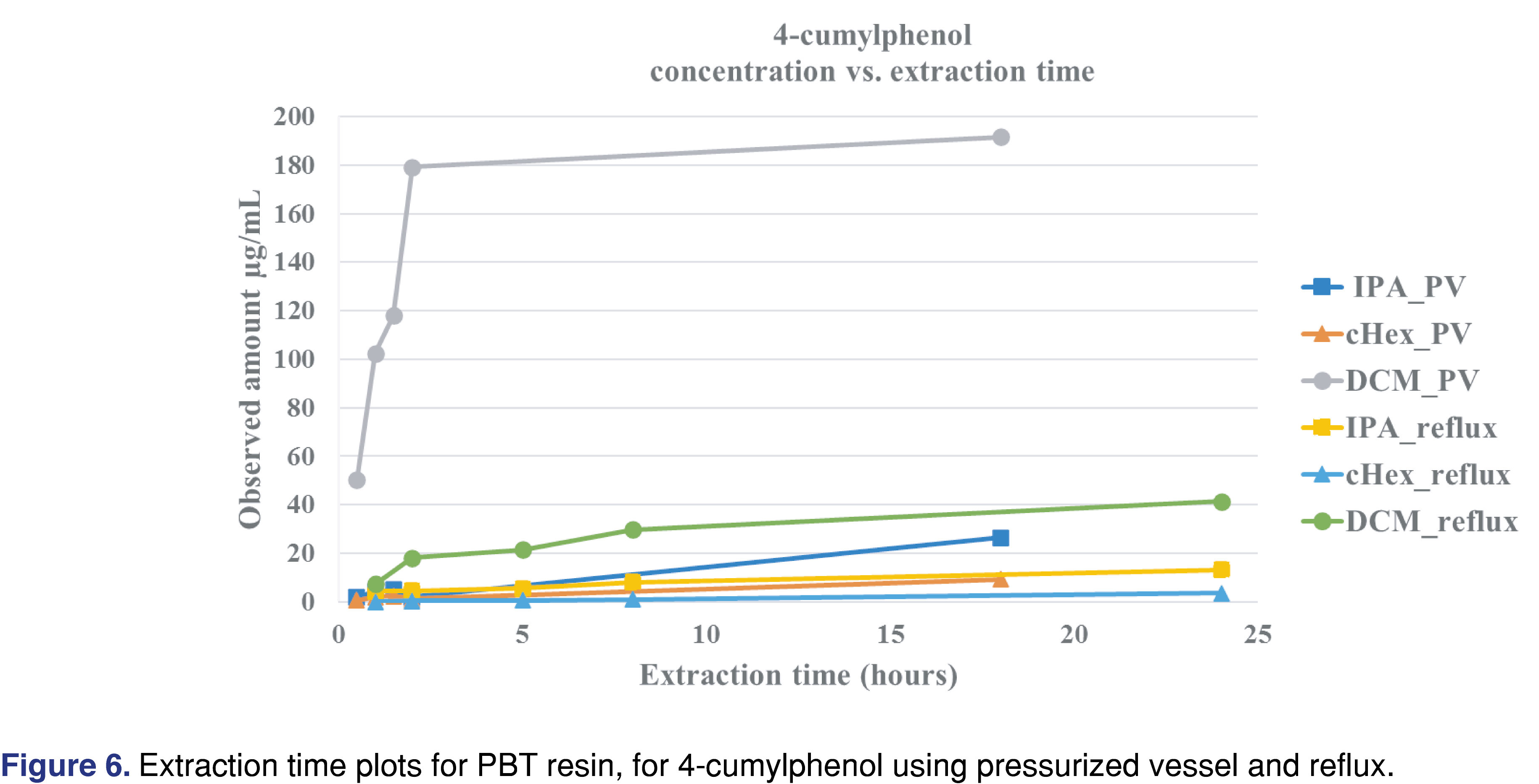
Figure 6 shows that the observed level of 4-cumylphenol was highest when DCM was used for the extraction, and the asymptotic level was reached in about 2 hours in the pressurized vessel, while over 8 hours of reflux extraction was required to approach equilibrium. Other solvents required significantly longer extraction times to reach equilibrium or to approach the levels observed in the DCM extracts. The logP of the different observed extractables are presented in Table 4 and show that acetophenone has the lowest logP. The logP values may play an important role when an extraction study is designed for a particular analyte. For an extractable with a lower logP value, higher levels may be observed in polar solvents than in apolar solvents. IPA is a more effective extraction media for acetophenone for all the extraction techniques than cHex, which is a non-polar solvent (Table 7). Overall, DCM extracted the highest levels of extractable compounds.
The level of observed BPA was consistently highest when pressurized vessel extraction was used. The pressurized vessel uses high temperatures, accelerating the extraction process; however, this condition also contributes to forming a known polymer degradation product, associated with polycarbonate. BPA was observed at much lower levels in reflux extraction and shaking conditions. This observation highlights the importance of using the proper extraction conditions for specific polymers to avoid falsely reporting high levels of an extractable that could instead be a by-product of the extraction process. The results of this extraction study are summarized in Table 7.
3.6 Analytical results for chlorobutyl rubber septa
Chlorobutyl rubber is one of the most commonly used packaging materials for closure systems, as it is inert and an excellent barrier to oxygen and moisture. Sterilized chlorobutyl rubber grey septa (West 4432/50 coated with BH2-40 and Flurotec (P/N 19700022 WPS S10-F451 4432/50G B2-40WESTARRS1M/PK)) were used for the study. The rubber elastomer shows very little (almost negligible) swelling in IPA and a high degree of swelling in cHex and DCM (Table 3). The observed swelling for the intact PTFE-coated stopper was 31.7% for cHex and 21.4 % for DCM. The swelling had a high impact on the solvent recovery for the pressurized vessel extraction: the rubber elastomer absorbed the entire volume of both DCM and cHex at the 18-hour time point. Because no solvent was recovered, the pressure vessel extraction results are presented for only 2 hours of extraction. The two primary extractables for all the solvents and all the conditions were the C21H40 butyl oligomer and BHT. Data for the two primary extractables and three more observed extractables are presented in Table 8.
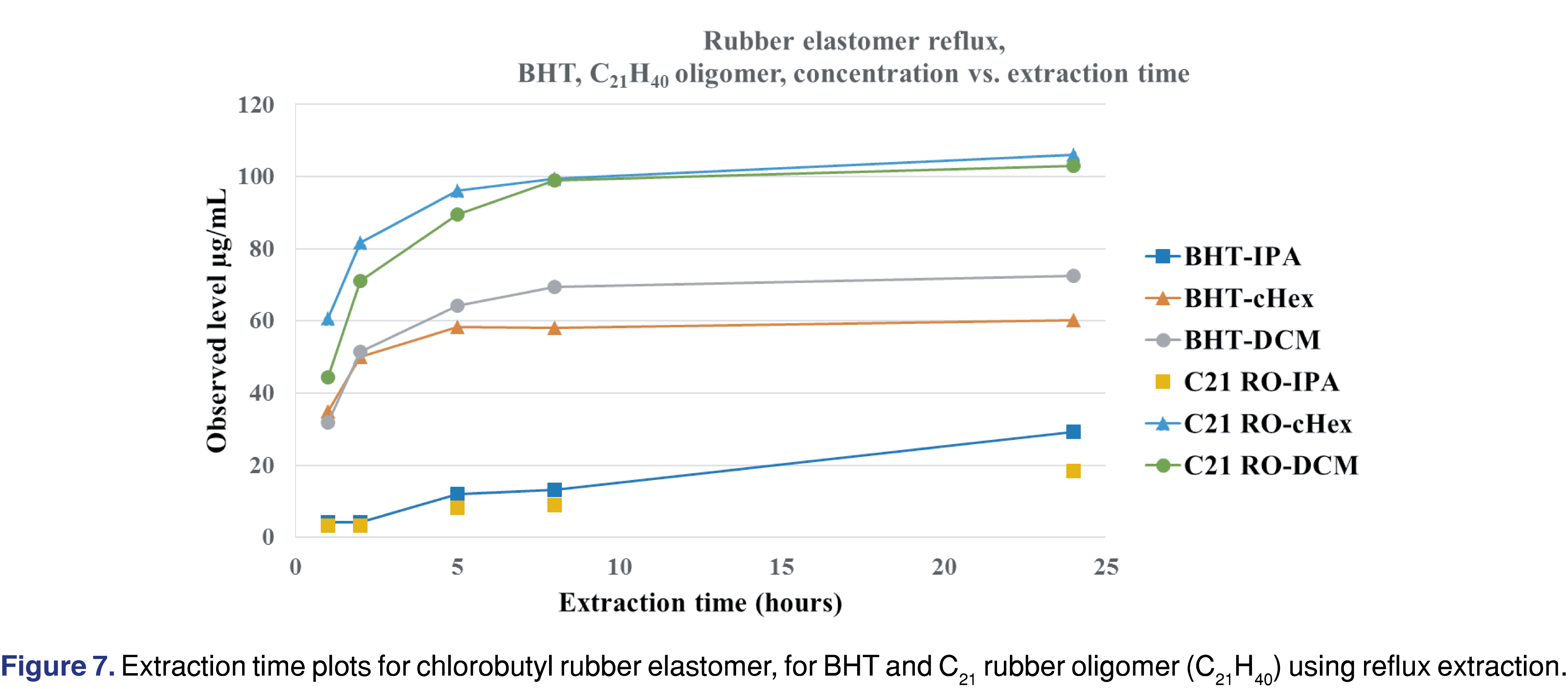
Figure 7 shows that for DCM and cHex extraction solvents, the BHT and C21H40 butyl oligomer levels reach the maximum observed concentration after 8 hours of reflux extraction, while for IPA, the observed levels are much lower, and the rate of extraction is much slower. The slower extraction rate and the lower observed levels of IPA make it difficult to justify using IPA as the only extraction solvent to mimic apolar drug formulations (i.e., lipids, highly organic emulsions, and polymeric excipients). IPA is a good and justifiable solvent to simulate the extraction power associated with polar drug formulations or when the formulation contains ethanol or isopropanol as the excipient.
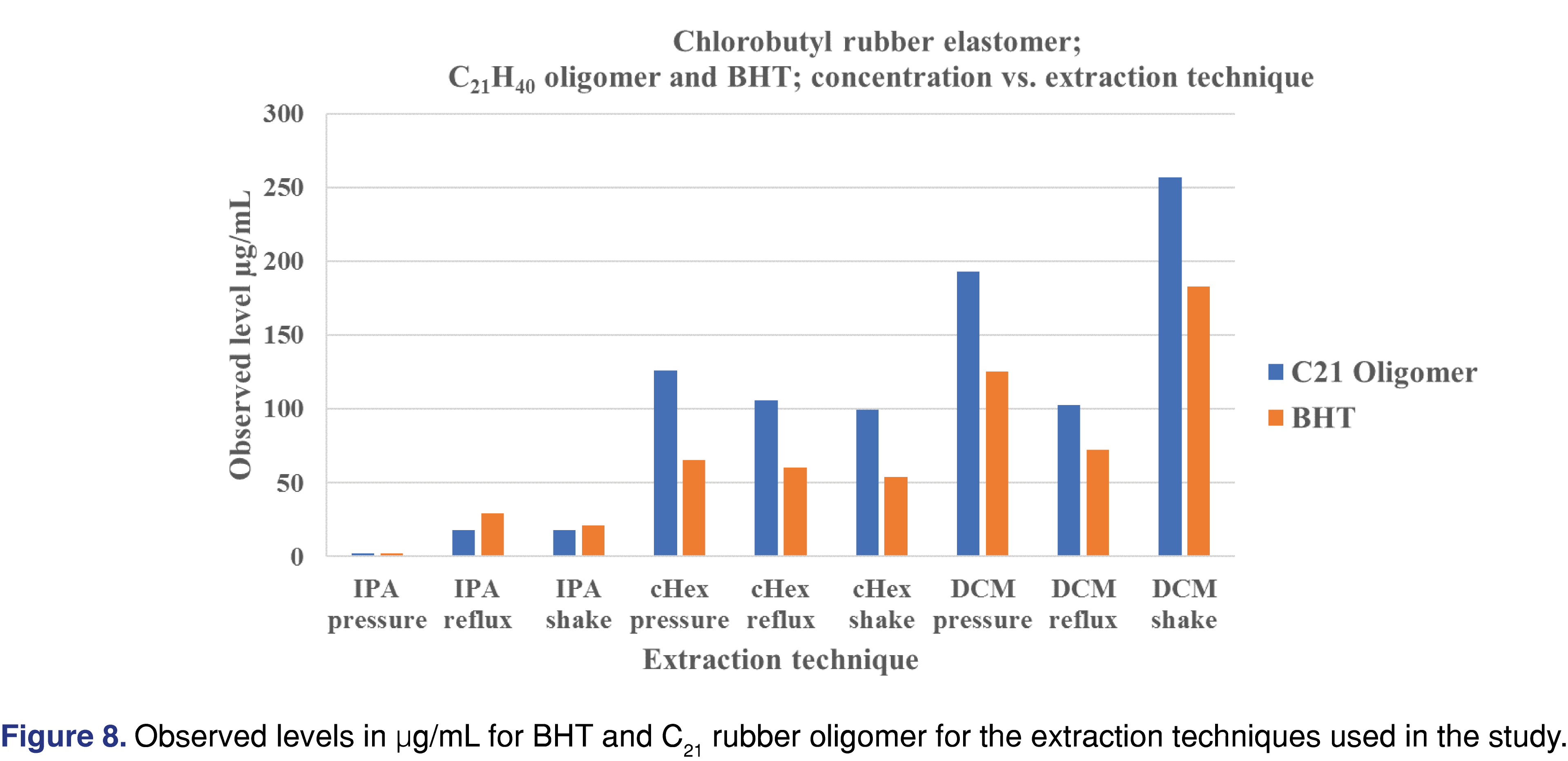
Figure 8 summarizes the extraction techniques for the C21 butyl oligomer and BHT. cHex provided the narrowest distribution of observed levels of the three extraction techniques, and IPA resulted in the lowest observed levels compared to DCM and cHex.
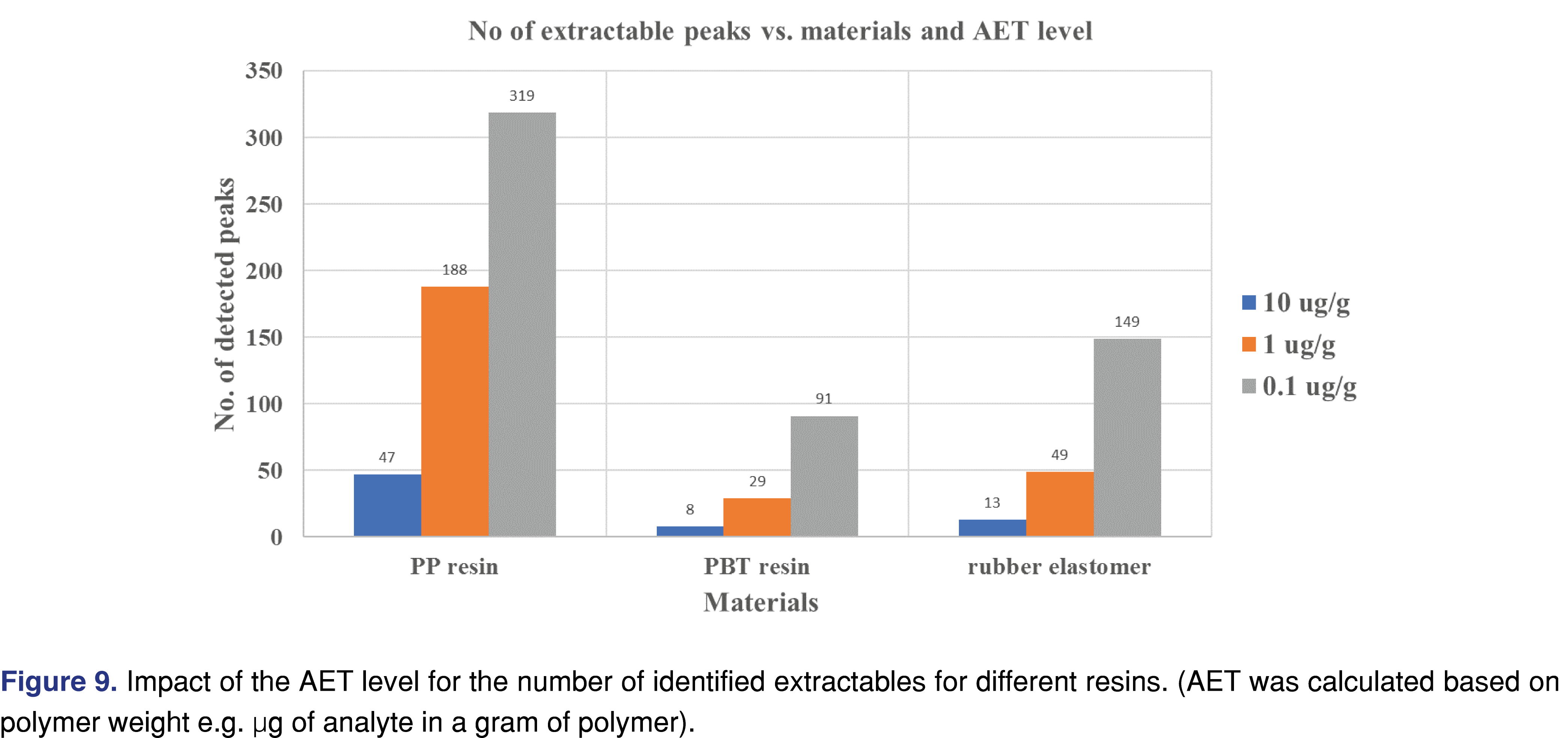
3.7 Impact of the analytical evaluation threshold (AET) level for the number of extractable species
It is well known amongst laboratories, engaged with E&L assessments that lowering the analytical evaluation threshold (AET) for the study results in a larger number of extractable analytes to be reported. This phenomenon has become a more significant issue for the E&L industry with the raised demands for testing large volumes of parenterals with associated low AET levels. Multiple graphical representations of the lower AET impact have been presented [30], providing a good summary of the issue. However, it is very difficult to find data related to this challenge. The low AET is associated with multiple challenges for laboratories, and in extreme cases, the level of evaluation can be below the detection capability of the analytical system. Beyond a few extreme cases (which can be managed using a state-of-the-art resource-consuming sample preparation), the underlying issue is that those low-level peaks show a high spectral background [31], making them difficult to identify. The graphical plot in Figure 9 shows the number of peaks present at or above the AET level for the three materials evaluated in this study using DCM as an extraction solvent. All three materials showed a similar trend when the AET level was reduced from 10 µg/g to 0.1 µg/g level (100 fold): the number of peaks increased 6.8 fold for the PP resin and 11.5 fold for the rubber stopper.
4.0. Conclusion
Overall, this study provides another view into the complexity of extractable studies. The successful execution of these studies requires appropriate design, with solvent selection and extraction conditions among the critical aspects.
Data from different polymeric materials were generated using different extraction solvents and extraction techniques focused on GC-MS-amenable volatile and semi-volatile extractables. The data show that the time required to reach asymptotic levels differs for each solvent and extraction technique. This observation correlates with a study published earlier [13], where data have been provided for a different polymer using various extraction techniques from different laboratories. Based on this limited data, solvents and extraction techniques are not equivalent, and extraction techniques and conditions for each solvent need some optimization.
These data also allow us to conclude that pressurized vessel extraction requires the shortest extraction time, which is useful for laboratories where many extractions have to be performed in a short time. However, due to the high pressure and temperature settings, the level of unwanted degradation products may be elevated compared to other techniques. These data also show a reasonable correlation between the solvent swelling capability and its extraction efficiency but found only a weak correlation between the dielectric constant and the extraction efficiency of the solvents. This paper presents data illustrating the limitations of ethanol and methanol as extraction solvents due to the formation of ethyl and methyl esters. As expected, the results of this study show differences in volatile extractables between the extraction solvents, and significant differences were also observed between the extraction techniques. It is challenging to draw a general conclusion or recommend a standardized test based on this data. This study suggests that a science-based “fit for purpose” approach for standardized testing rather than a “one size fits all” strategy needs to be implemented.
5.0 References
1. United States Pharmacopeia General Chapter <661> Plastic Packaging Systems and Their Materials of Construction, USP 43-NF 38. Currently Official on 28-Sep-2021.
2. Barth HG, Majors R. Approaches for Extracting and Determining Additives, Contaminants, and Low-Molecular-Weight By-Products in Synthetic Polymers. LCGC North America 31 (1), 14-29 (2013).
3. Lübtow MM, Haider MS, Kirsch M, Klisch S, Luxenhofer R. Like Dissolves Like? A Comprehensive Evaluation of Partial Solubility Parameters to Predict Polymer-Drug Compatibility in Ultrahigh Drug-Loaded Polymer Micelles. Biomacromolecules 20(8), 3041-3056 (2019).
https://doi.org/10.1021/acs.biomac.9b00618
4. Ahmad I et al. Effect of solvent polarity on the extraction of components of pharmaceutical plastic containers Pak. J Pharm Sci 30(1) (Suppl), 247-252 (2017).
5. Wolf BA. Solubility of Polymers. Pure AppL Chem 57(2), 323-336 (1985).
https://doi.org/10.1351/pac198557020323
6. Jenke D. Compatibility of Pharmaceutical Products and Contact Materials. John Wiley and Sons, Hoboken NJ, (2009).
https://doi.org/10.1002/9780470459416
7. Ball DJ, Norwood DL, Stults CL, Nagao LM. Leachables and Extractables Handbook, John Wiley and Sons, Hoboken NJ (2012).
https://doi.org/10.1002/9781118147672
8. Watabe AI et al. Approaches to Quality Risk Management When Using Single-Use Systems in the Manufacture of Biologics. AAPS PharmSciTech 16(5), 993-1001 (2015).
https://doi.org/10.1208/s12249-015-0368-z
9. ISO 10993-18:2020(E), Biological evaluation of medical devices -Part 18: Chemical characterization of medical device materials within a risk management process
10. United States Pharmacopeia General Chapter <1663> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems, USP 43-NF 38. Currently Official on 28-Sep-2021.
11. United States Pharmacopeia General Chapter <1664> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems, USP 43-NF 38. Currently Official on 28-Sep-2021.
12. Guidance for Industry Container Closure Systems for Packaging Human Drugs and Biologics, Chemistry, Manufacturing, and Controls Documentation, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) (1999).
13. Teasdale A et al. Controlled Extraction Studies Applied to Polyvinyl Chloride and Polyethylene Materials: Conclusions from the ELSIE Controlled Extraction Pilot Study. AAPS Pharm Sci Tech (2014).
https://doi.org/10.1208/s12249-014-0249-x
14. García ND et al. Large-Scale Assessment of Extractables and Leachables in Single- Use Bags for Biomanufacturing. Anal Chem 90, 9006-9015 (2018).
https://doi.org/10.1021/acs.analchem.8b01208
15. Stults CL, Ansell J, Shaw AJ, Nagao LM. Evaluation of extractables in processed and unprocessed polymer materials used for pharmaceutical applications. AAPS Pharm Sci Tech 16(1), 150-164 (2015).
https://doi.org/10.1208/s12249-014-0188-6
16. Jenke D et al. Extractables characterization of five materials of construction of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Tech 67(5), 448-511 (2013).
https://doi.org/10.5731/pdajpst.2013.00933
17. Heise T. Sawyer AY, Hirai T, Schaible S, Sy H, Wickramasekara S. Report on investigation of ISO 10993-12 extraction conditions, Regulatory Toxicology and Pharmacology 131, 105164 (2022).
https://doi.org/10.1016/j.yrtph.2022.105164
18. International Standard ISO 10993-12 fifth edition, Biological evaluation of medical devices – Part 12: Sample preparation and reference materials. Reference number ISO 10993-12:2021(E) (2021).
19. Rahi M, Smith M. Biocompatibility Failure and Solvent Effects on Chemical Characterization. Available at https://www.mddionline.com/testing/biocompatibility-failure-and-solvent-effects-chemical-characterization
20. Beißmann S, Stiftinger M, Grabmayer K, Wallner G, Nitsche D, Buchberger W. Monitoring the degradation of stabilization systems in polypropylene during accelerated aging tests by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry. Polym Degrad Stab 98, 1655-1661 (2013).
https://doi.org/10.1016/j.polymdegradstab.2013.06.015
21. Armstrong BL, Senyurt AF, Narayan V, Wang X, Alquier A, Vas G. Stir bar sorptive extraction combined with GC-MS/MS for determination of low level leachable components from implantable medical devices. J Pharm Biomed Anal 74, 162- 170 (2013).
https://doi.org/10.1016/j.jpba.2012.10.019
22. Alegre MR, Romero JE, Broch SC. Is it really necessary to validate an analytical method or not? That is the question. J Chrom A 1232, 101- 109 (2012).
https://doi.org/10.1016/j.chroma.2011.10.050
23. Ding W, Madsen G, Mahajan E, O’Connor S, Wong K. Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing. Pharm Eng 34 (6), 1-11 (2014).
24. Gugliuzza A. Solvent Swollen Polymer. In: Drioli E., Giorno L. (eds) Encyclopedia of Membranes. Springer, Berlin, Heidelberg. (2016).
https://doi.org/10.1007/978-3-662-44324-8_1407
25. Sienkiewicz A et al. Swelling effects in cross-linked polymers by thermogravimetry. J Therm Anal Calorim 130, 85-93 (2017).
https://doi.org/10.1007/s10973-017-6131-9
26. Edirisinghe PD, Barge VJ, Reichert M. Moving towards near perfect extractable study: Novel approach to justify model solvent selection and new screening tools for extractables. Smithers Rapra E&L US meeting June 29-30, (2021).
27.
28. Kelly M, Macdougall K, Olabisi O, McGuire N. In vivo response to polypropylene following implantation in animal models: a review of biocompatibility. Int Urogynecol J 28, 171-180 (2017).
https://doi.org/10.1007/s00192-016-3029-1
29. https://pubchem.ncbi.nlm.nih.gov/compound/4-Cumylphenol#section=Use-and-Manufacturing
30. Paskiet D, Jenke D, Ball D, Houston C, Norwood DL, Markovic I. The Product Quality Research Institute (PQRI) Leachables and Extractables Working Group Initiatives for Parenteral and Ophthalmic Drug Product (PODP). PDA J Pharm Sci and Tech 67, 430-447 (2013).
https://doi.org/10.5731/pdajpst.2013.00936
31. Vas Gy. Evaluation of Vitamin E Acetate Volatile Degradation Products; a possible Connection to the EVALI Epidemic. Rev Sep Sci 3(1), e21004 (2021).
https://doi.org/10.17145/rss.21.004
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License